Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in water. They help your body regulate chemical reactions, maintain the balance between fluids inside and outside your cells, and more. They’re also a key way to diagnose a wide range of medical conditions and diseases.
Electrolytes are substances that have a natural positive or negative electrical charge when dissolved in water. An adult's body is about 60% water, which means nearly every fluid and cell in your body contains electrolytes. They help your body regulate chemical reactions, maintain the balance between fluids inside and outside your cells, and more.
Your body gets electrolytes or their components from what you eat and drink. Your kidneys filter excess electrolytes out of your body and into your urine. You also lose electrolytes when you sweat.
Key terms to know:
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Your cells use electrolytes to conduct electrical charges, which is how your muscles contract. Those same electrical charges also help with chemical reactions, especially when it comes to hydration and the balance of fluids inside and outside of cells.
The key principle that electrolytes rely on is that certain chemical elements can naturally hold a positive or a negative electrical charge. When those elements are dissolved in a liquid, that liquid can then conduct electricity.
An example of this is salt water, which conducts electricity easily. Salt consists of sodium (positively charged) and chlorine (negatively charged), and when combined, their charges balance each other out. Atoms with an electrical charge are called ions (positive ions are called cations, while negative ions are called anions).
Dissolving salt in water splits the sodium and chlorine atoms apart, which means they go back to being positively and negatively charged. Electricity jumps between the sodium and chlorine ions — not the water molecules — because they have opposite electrical charges.
At the most basic chemical level, electrolytes help your body maintain balance. Just like electricity uses ions to travel from place to place in salt water, your body uses ions to transport chemical compounds in and out of cells.
There are several key elements that your body needs to maintain normal electrolyte levels. The following section includes the major elements, marked as positive (+) or negative (-), and what happens when there’s too much or too little of that element.
Sodium plays a critical role in helping your cells maintain the right balance of fluid. It’s also used to help cells absorb nutrients. It’s the most abundant electrolyte ion found in the body.
Magnesium helps your cells as they turn nutrients into energy. Your brain and muscles rely heavily on magnesium to do their job.
Your cells use potassium alongside sodium. When a sodium ion enters a cell, a potassium ion leaves, and vice versa. Potassium is also especially critical to your heart function. Too much or too little can cause serious heart problems.
Calcium is a key element in your body, but it does more than just build strong bones and teeth. It’s also used to control your muscles, transmit signals in your nerves, manage your heart rhythm and more. Having too much or too little calcium in your blood can cause a wide range of symptoms across different systems in your body.
Chloride (the name for a chlorine ion) is the second-most abundant ion in the body. It’s also a key part of how your cells maintain their internal and external balance of fluid. It also plays a role in maintaining the body’s natural pH balance.
This can cause acidosis, which is when your blood’s acidity is too high. It results in nausea, vomiting and fatigue, as well as rapid, deeper breathing and confusion. This usually happens in connection with too much or too little potassium.
Phosphate is a phosphorous-based molecule that’s a key part of transporting chemical compounds and molecules outside your cells. It helps your cells metabolize nutrients, and it’s also a key part of molecules called nucleotides, which are the building blocks that make up your DNA.
Not all the carbon dioxide that your body makes gets sent to your lungs for you to breathe it out. Instead, some gets recycled into bicarbonate, which your body uses to keep your blood pH levels normal.
Advertisement
Electrolyte problems are detectable using several different varieties of lab tests. Testing usually involves a broader type of test called a metabolic panel. If those results are abnormal, your healthcare provider may order follow-up tests, which can narrow down what’s causing the electrolyte imbalances. These follow-up tests are critical, as the specific cause of an electrolyte imbalance may need a specific type of treatment that won’t work for other causes.
Broader tests that can detect electrolyte problems include the following blood tests:
This test looks at several different processes in your body and shows data related to:
This test is similar to the basic metabolic panel but with additional data gathered. The additional items gathered include:
This is a broader test like the above metabolic panels, but it only looks for electrolytes. The electrolytes analyzed include sodium, chloride, potassium and bicarbonate.
Tests that are more specific for electrolyte problems include:
Most lab results include your result number and a reference range. A reference range has an upper and a lower limit, and any result that falls between the two is considered a “normal” result. Most of these results are communicated as "how much of a substance can be found in a specific sample size."
Mass is a unit of “how much.” It’s not the same as weight. The units of mass that are used are millimoles, milliequivalents or milligrams.
The prefix “milli-" means “1/1,000th.” The units of volume are usually displayed as a liter or fractions of a liter, like a deciliter (dL, which is 1/10th of a liter) or milliliter (mL, which is 1/1,000th of a liter).
Advertisement
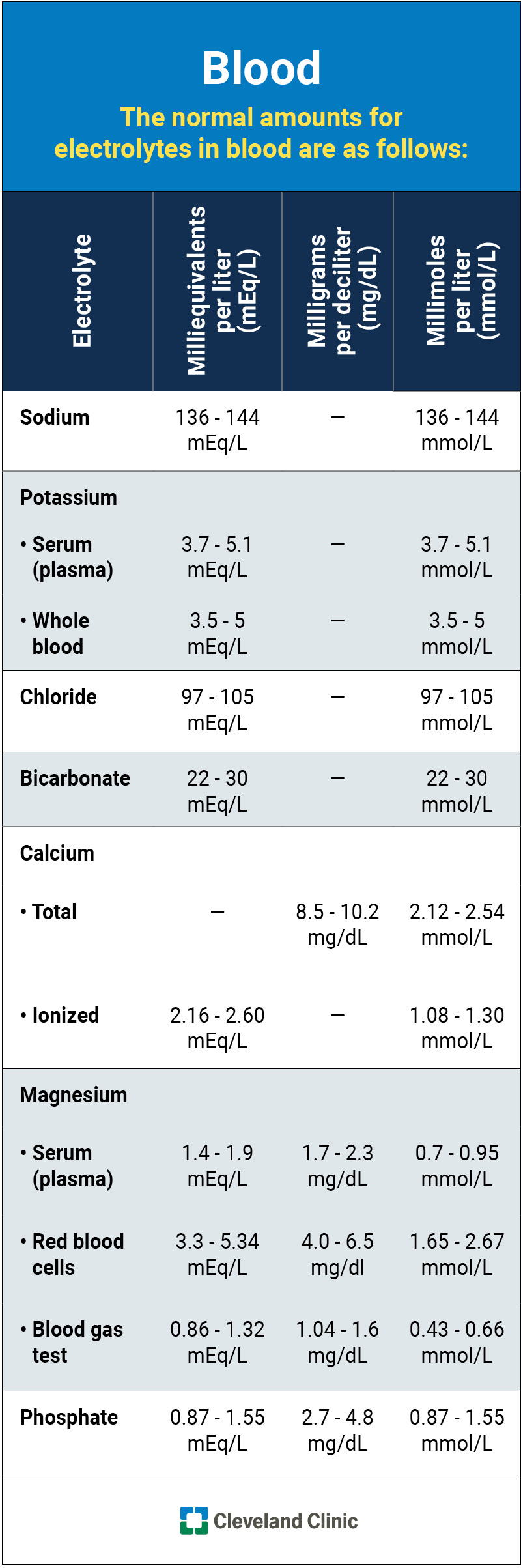
This infographic lists the normal (typical) ranges for each electrolyte in your blood.
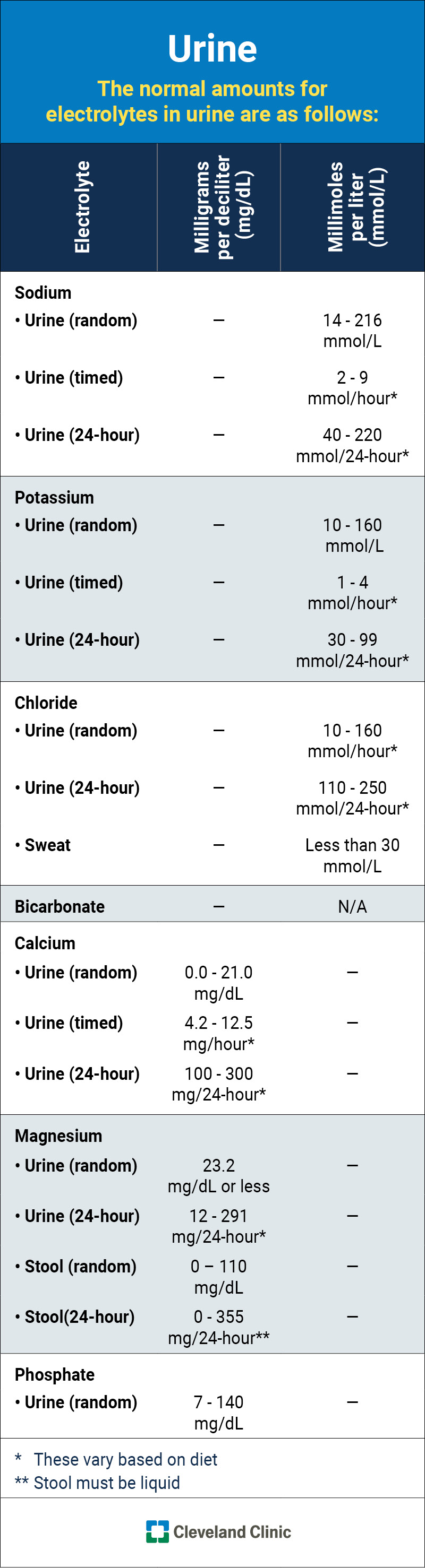
This infographic lists the normal (typical) ranges for each electrolyte in your urine.
Because everyone is different, sometimes you may have a result that falls outside the reference range. In other cases, you may have a normal result, but symptoms you're having and other test results will show you have a health problem.
One way to understand it is to think of the carnival trick where an entertainer spins a plate on the end of a wooden stick. If the plate leans too far in any direction, it will fall off, so balance is crucial. Your electrolyte levels and lab results rely on a similar balancing act, and your body is always trying to keep things as balanced as possible. Your body may be masking a problem by compensating with another body system or process. If your medical provider runs more than one test, they are likely making sure that your body isn’t hiding one problem by creating another.
Your healthcare provider or their staff can tell you when to expect your test results back.
If you don't understand your test results or have a result that isn't within the reference range and you have questions or concerns, you should call your healthcare provider. You should also call your healthcare provider if you notice a sudden change in any symptoms related to any test done on your electrolyte levels.
A note from Cleveland Clinic
Electrolytes are an essential part of how your body functions, affecting everything from hydration to how your heart beats. They can also help doctors diagnose a wide range of medical conditions and problems. Understanding electrolytes and the potential concerns that surround them can help you care for yourself, and help you avoid future health concerns. That way, you can take charge of your electrolytes and keep them from negatively affecting your life and routine.
Last reviewed on 09/24/2021.
Learn more about the Health Library and our editorial process.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy